The Periodic Table
Learning Objectives
Introduction
This unit addresses the varying physical and chemical properties of different elements. and the principles underpinning the Mendeleev Periodic Table.
Before you go ANY further, take this baseline assessment.
The Periodic Table is a chart listing all the known elements arranged in order of Atomic Number and in columns of elements with similar properties.
Terminology
You don’t have to know all these terms but this will be a useful place to check for a quick and simple definition throughout this unit. These are terms you will come across regularly for this topic.
Periodic Table (noun)
A table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns.
Mendeleev (noun (name))
Russian chemist who developed a periodic table of the chemical elements and predicted the discovery of several new elements (1834-1907)
Period (noun)
The horizontal rows of the periodic table are called periods.
Group (noun)
A column in the periodic table of the chemical elements.
Metal (noun)
Any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility (the ability to be hammered thin or stretched into wire without breaking), and high reflectivity of light.
Oxide (noun)
Any of a large and important class of chemical compounds in which oxygen is combined with another element.
Atom (noun)
Element (noun)
One of a class of substances that cannot be separated into simpler substances by chemical means. It is made of molecules or atoms of the same kind.
Compound (noun)
A pure substance composed of two or more elements.
Molecule (noun)
The smallest physical unit of an element or compound, consisting of two or more like atoms in an element and two or more different atoms in a compound.
Nucleus (noun)
The positively charged dense region at the centre of an atom, composed of protons and neutrons, about which electrons orbit.
Proton (noun)
One of the three basic particles in the atom, found in the nucleus with the neutron. It has positive charge. The number of protons in an atom determines the element. Carbon atoms will always have 6 protons.
Neutron (noun)
One of the three basic particles in an atom. It is found in the nucleus and has zero charge. An atom, such as a carbon atom, could have different numbers of neutrons.
Electron (noun)
One of the three basic subatomic particles. It is very light and orbits round the nucleus of an atom. It has a negative charge.
Ion (noun)
An electrically charged atom or group of atoms.
Session One – About The Periodic Table
Mendeleev
The modern Periodic Table is based on an earlier Periodic Table made by a scientist called Mendeleev.
Dmitri Ivanovich Mendeleev, Russian, 8 February 1834 – 2 February 1907, was a Russian chemist and inventor. He is best known for formulating the Periodic Law and creating a version of the periodic table of elements.
Others had tried to arrange all the elements before, but Mendeleev was the first to correctly arrange it.
He arranged it by both Relative Atomic Mass and chemical properties with gaps where elements were later discovered.
The modern Periodic Table
The modern Periodic Table is only slightly different and is arranged by Atomic Number and chemical properties.
The columns of the Periodic Table are called groups. The rows of the Periodic Table are called periods.
Elements on the Periodic Table are written with their Chemical Symbols and two numbers representing their Relative Atomic Mass and Atomic Number.
NOTE: The two bottom rows – the lanthanides (57-71) and the actinides (89-103) fit into the two blank spaces in the upper chart. They are expanded out.
Activity One:
Print out the periodic table and create your own colour key to identify the different element types:
Note: they are not always identified by the same names / groups. Don’t worry if you see other words used for some of the groups from time to time.
- non-metal
- noble gas
- alkali metal
- alkaline earth metal
- metalloid
- halogen
- post-transition metal
- transition metal
- rare Earth metal: lanthanides
- rare Earth metal: actinides
Use this to help you:
Session Two – History of The Periodic Table
Scientists always try to look for patterns to help arrange complicated problems. Many different elements had been discovered so scientists wanted to arrange them based on patterns they noticed. Some scientists arranged them by patterns in chemical properties while others tried to arrange them by patterns in physical properties like melting point or relative atomic mass.
In 1808 a scientist called John Dalton, who created the Dalton Model of the atom, arranged elements by their relative atomic mass.
In 1864 a scientist called John Newlands arranged elements by relative atomic mass but because he noticed that ever 8th element had similar chemical properties he arranged them into what he called ‘octaves’.
In 1869 Mendeleev was the first to place gaps where the elements did not fit the pattern but still arranged them by both their chemical properties and relative atomic mass. This enabled Mendeleev to predict the existence of elements that had not yet been discovered.
There were some problems with Mendeleev’s Periodic Table. One such problem was that Argon has a greater relative atomic mass than Potassium which would put Argon in the Alkali Metals.
Once the structure of the atom was investigated by physicists in the early 1900s it was discovered how to correct Mendeleev’s Periodic Table by ordering the elements by atomic number instead of relative atomic mass.
Atomic number mean the order of increasing number of protons in the nucleus. The number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number
This produced the modern Periodic Table.
Activity Two:
Play the Period Table Game here.
Screen shot and print out your results!
Remember – The number of protons is the atomic number
Session Three – Groups
Groups
Groups are the columns of the periodic table.
The elements are arranged groups of similar chemical properties.
Elements have similar chemical properties when they have the same number of electrons in the Outer Shell.
Trends within Groups
The chemical properties of elements within a group are similar.
The reactivity within a group changes as you move up or down the periods (rows).
Examples:
- Group 1: The Alkali Metals all react strongly with water.
- The reactivity increases as you go down the group.
- Group 2: The Alkali Earth Metals all react strongly with steam and acids.
- The reactivity increases as you go down the group.
- Group 7: The Halogens all act as bleaching agents and kill bacteria.
- The reactivity decreases as you go down the group.
- Group 0: The Noble Gases are all inert (unreactive).
Periodic Laws & Families (Groups)
Alkali Metals
- found in group 1
- highly reactive metals
- do not occur freely in nature
- only one electron in their outer shell
- malleable
- ductile,
- good conductors of heat and electricity
- softer than most other metals
Alkaline Earth Metals
- found in the second group
- very reactive
Transition Metals
- 38 elements in groups 3 through 12
- ductile
- malleable
- conduct electricity and heat
Post-Transition Metal
- The “other metals”
- located in groups 13, 14, and 15
- ductile
- malleable
- solid
- opaque
Metalloid
- boundary that distinguishes metals from non-metals
- properties of both metals and non-metals
Non-Metal
- groups 14-16
- not able to conduct electricity or heat very well
- very brittle
- can be gases and solids
- no metallic luster
- do not reflect light
Noble Gas
- group 18
- 8 electrons in their outer shell
- stable
- highly non-reactive
Halogen
- five non-metallic elements found in group 17
- 7 electrons in their outer shells
- go from gas->liquid->solid at room temperature as you descend the group
Rare Earth Metal: Lanthanides
- elements in the f-block in the sixth period
- soft metals
- all naturally occur except for Promethium
- useful for their metallurgical properties in alloy form
Rare Earth Metal: Actinides
- elements in the f-block in the seventh period
- radioactive
- most are synthetic, that is, human-made
- silvery or silvery-white luster in metallic form.
Activity Three – Properties:
Session Four – Periods
Periods
- The elements in each row of the Periodic Table all have the same number of Electron Shells.
- These rows are called periods.
- The periods are arranged by the number of Electron Shells.
Electron Shells
The electrons of an atom orbit the nucleus in a fuzzy cloud.
As an atom’s atomic number increases, its shells must accommodate an increasing number of electrons.
Example: Nickel has 28 atoms, giving it an atomic number of 28. To accommodate these 28 atoms it needs 4 shells. The elements with 4 shells are on the fourth row (period).

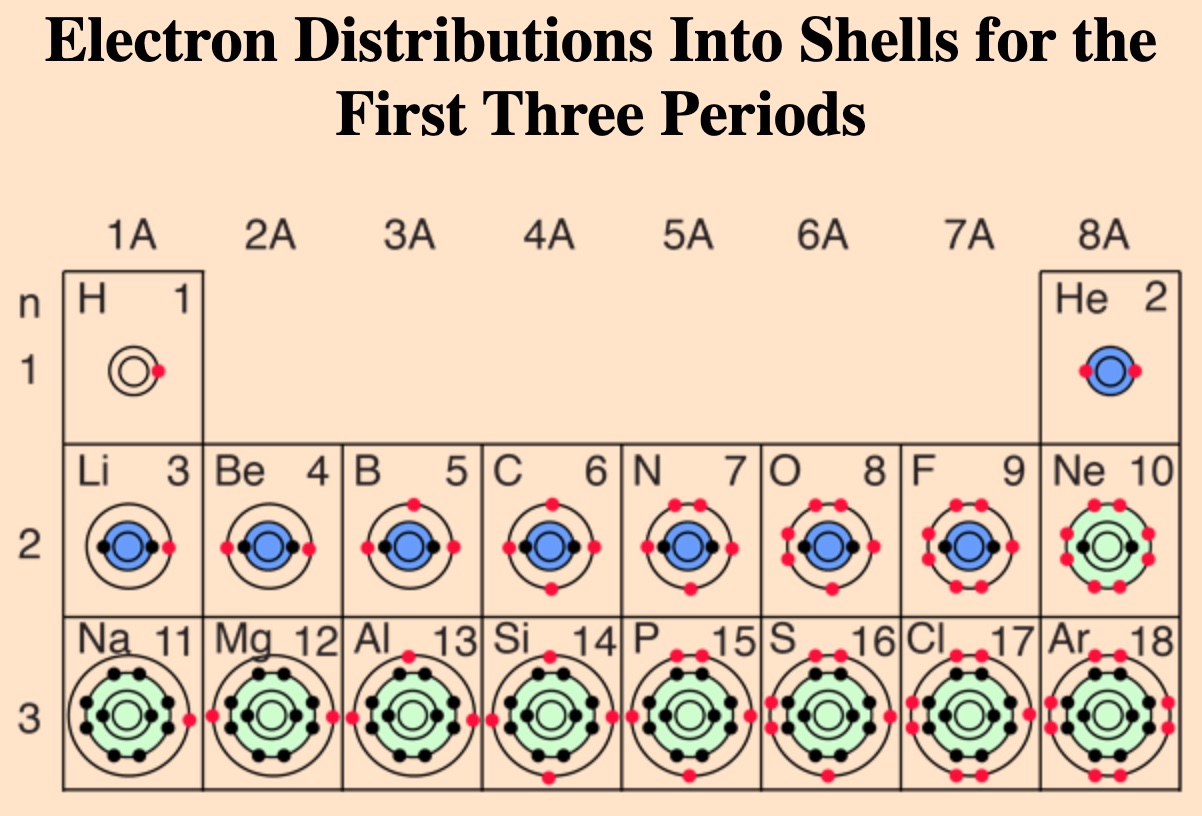
Trends within Periods
- The chemical and physical properties of elements change as you move along a period.
Activity Four:
Use this to study the types of elements and their uses.
Create your own period table element cards with images to show their possible use.
Use the colour coding from activity one to colour-code the cards in some way (background / letter colour / border etc…)
Periodic Table Deliverables.
- Activity One – Colour-coded periodic table
- Activity Two – Periodic Table Game Printouts
- Activity Three – Colour-coded periodic table by States
- Activity Four – Periodic Table element cards
Wrap up!
Attempt this game and see how far you get. Might knowing the properties of the elements help with this game?
Quiz.
Which element are you?
The properties of elements define where they site on the periodic table. Use this quiz to identify which properties suit you and which element you are.

