Welcome to Solids Day. It is day 2 in the series of Properties of Materials Days.
These are your objectives for today:
| a. You will compare and group together everyday materials on the basis of their properties, including their hardness, solubility, transparency, conductivity (electrical and thermal), and response to magnets. |
| b. You will give reasons, based on evidence from comparative and fair tests, for the particular uses of everyday materials, including metals, wood and plastic. |
| c. You will explain that some changes result in the formation of new materials, and that this kind of change is not usually reversible, including changes associated with burning and the action of acid on bicarbonate of soda.. |
Session One – Technical Stuff (a, b, c).
Properties of Solids

CHARACTERISTICS OF SOLIDS
One of the main characteristics of solids is that they hold their own shape. So if you put a solid in a container it won’t change its shape… No matter how much you move or slide it around. You can even grind a solid up so that it fills up a container. If you look at the powder under a microscope you will still see little tiny solids that you couldn’t change.
You know that liquids are different because if you put a liquid into a container it will fill it up as much of the container as it can. In the same way that a solid holds its shape the atoms inside of a solid are not allowed to move around much. This is a physical characteristic of all solids. It happens no matter how small the pieces are.
The atoms in liquids and gases move around in all directions. The solid atoms and molecules are trapped in their places. The atoms still spin and the electrons still move but the entire atom doesn’t go anywhere. They just kind of jiggle in place.
Every solid has properties along a scale, for example hardness. You cannot simply state that a solid material is hard or soft, but only that it it harder or softer than something else. To record the hardness property of a solid we would use a hardness scale.
Every activity today (except one – combustion) will lead you to categorize a property of a solid material / object. You need to remember – and this is IMPORTANT – that you are actually placing them on a scale.
TERMINOLOGY & DEFINITIONS
Metal – A metal is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well.
Wood – Wood is a porous and fibrous structural tissue found in the stems and roots of trees and other woody plants.
Plastic – Plastic is any of a group of synthetic or natural organic materials that may be shaped when soft and then hardened, including many types of resins.
Magnetism – A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets. 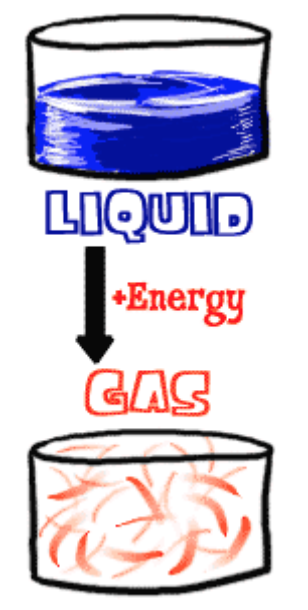
Electrical Conductivity – In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electrical current) in one or more directions.
Thermal Conductivity – The thermal conductivity of a material is a measure of its ability to conduct heat.
Insulation – Thermal insulation is the reduction of heat transfer between objects.
Resistance – Resistance is an electrical quantity that measures how material reduces the electric current flow through it.
Transparent – Transparency is the physical property of allowing the transmission of light through a material.
Opaque – Opacity is the property of blocking the transmission of light through a material.
Translucent – translucency permits light to pass through but diffuses it so that persons, objects, etc., on the opposite side are not clearly visible.
Combustion – Combustion, or burning, is a high-temperature chemical reaction between a fuel and oxygen (usually). It produces a mixture known as smoke. Combustion doesn’t always result in fire, but when it does, a flame is a usually seen.
Hardness – A measure of how easily a material can be scratched.
Comparative Test – comparative testing, is a process of measuring the properties or performance of products against each other.
Fair Test – a fair test means deciding what results you’ll be comparing, controlling variables, avoiding bias, and figuring out a way to distinguish chance differences from meaningful ones.
Reversible Change – a reversible change happens by a process in which a material can be returned to its original state.
Irreversible Change – an irreversible change happens by a process in which a material can NOT be returned to its original state.
Session Two – Magnetism (a, b)
WHAT METALS ARE MAGNETIC?
Objects that are made up of iron, cobalt, or nickel have magnetic properties. Common objects such as keys, nails, screws, fasteners, etc. are often made up of a combination of elements that have and do not have magnetic properties. Alloys (mixture of different metals) can have magnetic properties if they contain some iron, cobalt or nickel.
TESTING SOLIDS WITH A MAGNET
Materials:
plastic tray, large sheet of paper, black crayon, blue plastic spoon, blue rubber ball, blue wooden cube bead, blue Unifix cube, red wood golf tee, red pipe cleaner, red octagon jewel, red plastic button, Ping-Pong ball, small, white plastic spoon (taster spoon), plastic cup lid, steel ball, steel washer, steel nut, jumbo metal clip, brass washer, bobby pin, acrylic cube, acrylic cylinder, cork, wooden stirrer, and a magnifying glass.
Method:
Investigate which solids are attracted to magnets and those that are not.
Results:
Record your findings with the T Chart.
Conclusion:
Further Questions (answer in science notebook or on paper):
What did you discover about solids and their magnetic properties today?
Are all solids magnetic? Why or why not?”
Are nickels, pennies, dimes, quarters, half-dollars and dollar coins magnetic?
Explanation – WHAT MAKES A MAGNET?
Session Three – Conductivity (a, b)
Electrical Conductivity
Early scientists who worked with electricity found that current flows through some materials and not others. The electrical conductivity tester will allow you to identify those materials that are electrical conductors and those that are not conductors.
Materials you will need:
- Power Pack
- Bulb holder
- 3 volt flashlight bulb
- Number 22 gauge insulated wire with approximately 1″ of insulation stripped off both ends
Method:
Investigate which solid materials conduct electricity and those that do not.
1. Connect the positive side of your power pack to the bulb holder and another wire to the other side of the bulb holder. Your conductivity tester is now ready to be used. (Figure 1)
2. Gather different household items to determine if they conduct electricity. Some examples of items to try are pencils, paper, copper, screws, screw drivers, etc. (Figure 2)
3. Touch the ends of wires to the different objects. If the bulb lights, you have completed the circuit, showing that the object is a conductor. (Figure 3

Results:
Record your findings with the T Chart.
Conclusion:
You have gained knowledge about the materials tested which can be used as you design new ways to use electrical power.
Insulators like plastic are used in encasing electric wires in order to avoid exposure. Exposed electric wires can easily come into contact with water and cause a fire outbreak, or they may electrocute human beings and animals.
Concluding Question (answer in science notebook or on paper):


Explain how the understanding of electrical conductivity and insulation is used in the making of a light switch. Use diagrams to explain your points.
Thermal Conductivity – Performing a Heat Conduction Experiment With Hot Water
Some materials conduct heat better than others. This is a super important part of the cooking process.
Materials you will need:
- a cooking pot,
- a cold stick of butter or cold coconut oil (from the fridge),
- and three or more types of stick (objects such as long spoons, pipes etc – as long as they are all similar length and thickness) such as:
- wooden stirring spoon or stick,
- a plastic spoon or pipe,
- a regular metal spoon.
- stopwatch
(You need to get objects that are relatively long. For example, if you put a spoon in the pot the handle should be coming out of the pot by a few inches.
Method:
- Fill a cooking pot of any size about halfway full with water and place it on a normal stove burner. Boil water the same way you would if you were going to make spaghetti or pasta. While any pot will work, a shallow, broad pot might help you balance the butter on the spoons more easily.
- Take each of your three types of sticks, pipes, or spoons and lay them in the water handle down. You can place them next to each other or facing away from each other. The bowl portion of the spoon will be at a diagonal. Try to make that diagonal as close to horizontal as possible by leaning the end of the handle that is in the water up against the side of the pot.
- Cut three slices of butter or oil. You should cut them about a quarter inch each, but it isn’t that important – just that they are the same size. Now place one slice in each of the spoons or on the protruding end or the objects. Make sure they don’t slide into the water and don’t touch the objects!
- Start your stopwatch. Watch for a few minutes as the butter / oil melts. Look to see which melts frost, second, and so on and make a note of the times in which they start to melt and when they fully melt (if at all). Recording chart provided below.
Results:
Record your findings in the chart below (or one of your own).
Conclusion:
Thermal conductivity is actually about the conduction of heat or transfer of heat.
Concluding Question (answer in science notebook or on paper):
Explain how you think thermal conductivity is important in each example for cooking:
- Deep dish cooking pans (such as a bread pan)
- Oven glove
- Wooden spoon
- Sheet pan
- Aluminium foil
- Silicon Stirrer
- Coffee pot
Session Five – Transparency (a, b)
WHAT IS TRANSPARENCY?
The property or state of being transparent is a property which admits of the passage of: rays of light so that forms, colors, and brightness of objects can be seen through it. Opacity os the opposite meaning that rays of light can not pass through it. Translucency permits light to pass through but diffuses it so that persons, objects, etc., on the opposite side are not clearly visible. Translucency sits between transparency and opacity.
TESTING TRANSPARENCY WITH A LIGHT
Materials:
A variety of flat items such as: plastic lids, wooden boards, paper, card, tissue, glass lids and lenses, pan lids, lego base boards etc…
Large light source.
Table or flat surface covered with a dark cloth.
Darkened room (doesn’t need to be pitch black).
Method:
Investigate which items allow light to pass through.
Arrange the items in order of transparency.
Results:
Record your findings with this line diagram (or one of your own – you can write, draw, or use photos).
Explanation:
Conclusion:
Further Questions (answer in science notebook or on paper) – explain with diagrams and labels:
- What makes an object transparent?
- What makes an object translucent?
- What makes an object opaque?
Session Six – Combustion (b, c)
Changes to solids can be reversible or irreversible. If a piece of glass is heated, it becomes a liquid. When it cools, it becomes a solid again. It is glass again. It might not look the same. It might be a different shape for example, but it will have the same properties. The changes that happen when it is heated will be reversed when it is cooled.
If you heat wood it doesn’t melt, it burns. Burning is called combustion. Combustion, or burning, is a high-temperature chemical reaction between a fuel and oxygen (usually). Wood reacts with oxygen when it burns. The combustion of wood produces carbon dioxide and water, which are reaction products released as gases into the atmosphere.
Solid wood disappears when it burns, and it is converted into gas products, eventually leaving only ashes that consist of wood’s minor components that remain solid and do not burn.
The combustion of wood produces heat and light.
Once burned, the products of the burned wood can not be changed back into solid wood. This is an irreversible change. Not all changes to wood are irreversible. Combustion, however, is always an irreversible process.
Investigation:
Collect together some items to burn in a fire, for example:
- paper
- card
- fabric
- tissue
- leaves
- wood
- metal
- wax
(Try not to use anything plastic because of the toxic fumes).
Observe the combustion process carefully.
Questions (answer in science notebook or on paper):
- Which items burned most easily (most combustable)?
- Which items burned least easily (least combustable)?
- Did any items not burn? Why do you think this is?
- What did you observe of the product (what was left) after burning?
Session Six – Wrap Up – Categorization!
Use the following diagram to arrange solid objects by two properties (an example using a different property we have not covered is provided):
The properties to choose from are:
transparency
magnetism
electrical conductivity
thermal conductivity
Properties of Solids